Sputnik V: A Blaze of Hope As Vaccine Hits Indian Market This Week: Comparative Study With Covaxin, Covishield
SPUTNIK V vaccine:
“By the time the World Health Organization declared Covid-19 a pandemic in early March 2020, the Gamaleya National Center of Epidemiology and Microbiology in Moscow was already working on a prototype of Sputnik V, funded by the Russian Direct Investment Fund (RDIF), the country’s sovereign wealth fund,”
Sputnik V, which shares its name with the world’s first artificial satellite made by Russia, is an adenovirus-based vaccine. It has also been approved in 59 countries with a total population of over 1.5 billion people.
HOW DOES IT WORK?
The vaccine, also known as Gam-Covid-Vac, is a combination of two different adenoviruses (Ad26 and Ad5). The adenoviruses — viruses that cause common cold — are combined with the SARS-CoV-2 (the virus that causes Covid-19) spike protein, which prompts the body to make an immune response to it. Using the same adenovirus for the two doses could lead to the body developing an immune response against the vector and destroying it when the second dose is administered. Two different vectors reduce the chance of this.
The SARS-CoV-2 virus is studded with proteins that it uses to enter human cells. These so-called spike proteins make a tempting target for potential vaccines and treatments, Researchers added the gene for the coronavirus spike protein to Ad26 and Ad5, and engineered them so they could invade cells but not replicate.
What is ADENOVIRUS VACCINES?
“Sputnik V is a viral-vector vaccine. That means that it uses a modified version of a different virus as a tool to transport genetic material to a cell. Sputnik V was developed using adenoviruses, which normally causes respiratory infections, but other viruses (including the influenza or measles virus) have also been used in other viral-vector therapies,”
The virus, which is used as a vector, is altered so it poses no threat of causing an illness. The report added that it is inserted with an extra gene that is unique to the virus being targeted. For Covid-19 vaccines, this gene contains instructions on how to make a spike protein, which is found on the surface of the coronavirus. is a viral vector vaccine, similar to those developed by AstraZeneca and Johnson & Johnson (J&J). Vector vaccines are easier to manage than mRNA vaccines, which need to be stored at very low temperatures.
COMPARISON WITH COVISHIELD AND COVAXIN:
Covishield, the Oxford-AstraZeneca vaccine being manufactured by the Serum Institute of India, follows the same philosophy. It “is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees. It has been modified to look more like coronavirus – although it can’t cause illness,”
Covaxin, on the other hand, is an inactivated vaccine — which means that it is made up of killed coronaviruses, making it safe to be injected into the body. “Bharat Biotech used a sample of the coronavirus, isolated by India’s National Institute of Virology. When administered, immune cells can still recognise the dead virus, prompting the immune system to make antibodies against the pandemic virus,”
Covaxin works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus. “The antibodies attach to viral proteins, such as the so-called spike proteins that stud its surface,”
“Inactivated viruses have been used for over a century. Jonas Salk used them to create his polio vaccine in the 1950s, and they’re the bases for vaccines against other diseases including rabies and hepatitis A.”
EFFECTIVENESS OF SPUTNIK V:
President Putin surprised the world and the scientific community by announcing Russian approval for emergency use of Sputnik V as early as August 2020. Trail phases I and II results, was on 76 participants of an open, non-randomised trial, were published in the “Lancet” in September. According to the paper, all participants developed SARS-CoV-2 antibodies and that no serious adverse events were detected.
Interim phase III data were published in early February 2021. The randomised, double blind, placebo controlled trial included nearly 22,000 adults aged 18 years or older recruited through 25 hospitals and clinics in Moscow between 7 September and 24 November 2020. Each participant received either two doses of the vaccine, or a placebo, which were administered 21 days apart,
Interim results (based on data so far from 14 964 participants in the vaccine group and 4902 in the placebo group) indicate that the vaccine is 91.6% effective, based on its ability to prevent symptomatic infection, the paper added.
WHAT ARE THE RISKS?
In early April, the EU Observer claimed that four people had died and six others had experienced serious health complications after being vaccinated with Sputnik V. In response, Russia’s Federal Service for Surveillance in Healthcare, Roszdravnadzor, denied there was a direct link with the vaccine.
TO CONCLUDE:
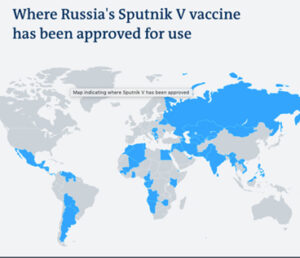 Sputnik V has been approved in 60 states, including India, Mexico, Iran, Ghana, Sri Lanka and Serbia, as well as in the Palestinian territories and Republika Srpska, in Bosnia-Herzegovina.
Sputnik V has been approved in 60 states, including India, Mexico, Iran, Ghana, Sri Lanka and Serbia, as well as in the Palestinian territories and Republika Srpska, in Bosnia-Herzegovina.
Though Sputnik V has yet to receive EMA approval, the EU member states Hungary and Slovakia have granted emergency national approvals. It is already being used in Hungary to vaccinate citizens, but in Slovakia the authorities have not yet approved use of the 200,000 doses delivered by Russia.
The efficacy of other vector vaccines such as AstraZeneca (76%) and J&J (85.4%), which only use one vector, is much lower than that of the mRNA vaccines by BioNTech-Pfizer (95%) and Moderna (94.1%). Use of two different vectors promises a higher vaccine efficacy (for Sputnik V), claiming an efficiency of 91.6% with no serious side-effects, gives a glimpse of hope in our struggling days fighting pandemic and vaccine crisis, as it (Russian Sputnik V) hits Indian Market this week.